Novel painkiller needs backing to reach kids in pain
When a doctor faces a child in pain, she has very few remedies to comfort her patient. None of them ideal. Now, a team from the Copenhagen Science City-partner University Hospital Rigshospitalet has developed a simple way to provide rapid pain-relief to young patients. Though still in development, it is remarkably close to market. It will take just three years and 50 million DKK to secure a permission to sell the novel pain treatment for children, so now the team is looking for investors.
Novel solution minimizes side effects
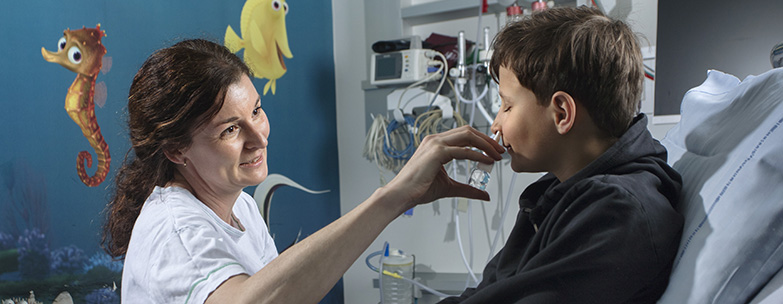
There are four common ways to relieve children of pain. You can inject the drug, and add to the kids’ discomfort. You can feed the child a liquid or tablet, and sit back for an hour until the drug takes effect. You can provide laughing gas but only if the child is old enough to use the required equipment. Finally, you can administer a sedative, which may relieve anxiety, but will not reduce pain. The need is there, and the novel alternative was literally under everyone’s nose, explains Bettina Nygaard Nielsen, who is part of the development team and co-founder of the new company Cessatech.
We discovered that combining a well-known painkiller with a familiar sedative work fast and well. Usually these drugs are injected. What we do, is to spray them up the child’s nose with a paediatric nasal spray device. This ensures reliable effect and minimizes side effects”: Bettina Nygaard Nielsen, pharmacist Ph.D. Rigshospitalet.
Rare go-ahead from authorities
Drug development is usually a multi-billion dollar effort spanning decades. However, both drugs in the nasal spray had individual approval for injection, so Bettina Nielsen and her colleagues argued to the European Medicines Agency that it should not take long to approve a feasible plan that could lead to approving their combination of drugs and spray-device. The EMA has now approved a so-called paediatric investigation plan. This lays out the clinical trials needed for the nasal spray to achieve a marketing authorisation within a few years and within a moderate budget.
“We are truly thrilled. An EMA approval of a development plan intended for a Paediatric-Use Marketing Authorisation is extremely rare. This really gives us hope, that in the near future many more children in Denmark and Europe may benefit from this new treatment”, says Bettina Nygaard Nielsen, pharmacist, Rigshospitalet
Ready-to-use solution may open market
Even though the comforting nasal spray cannot be marketed yet, staff at Rigshospitalet have used it successfully for five years, explains Dr. Steen Henneberg who has collaborated with Nielsen since the initial concept phase. Highly specialised hospitals have pharmacies that can supply the combined drugs to the clinic, where certified personnel can decant them into the spray device. Such a kit of parts is fine for the likes of Rigshospitalet but may not be feasible in other settings, says Henneberg, who has years of experience in treating pain in children.
I believe this procedure is too cumbersome for ambulances, emergency rooms and even most hospitals. A ready-to-use nasal spray is likely to be more feasible. An EMA approved product will have documented effect and safety, so I am sure that health care professionals working with children will add the nasal spray to their armoury”: Steen Henneberg MD Ph.D. senior consulting anaesthetist.
Investor could help children in pain soon
Once the team finds the backers they need, the clinical trials to document safety and efficacy should not take more than three years, states the teams’ commercial project manager Jes Trygved. During his career, he has been in charge of marketing several novel medicines at Danish pharma major Lundbeck. To him, this project is different.
We could help a lot of children in pain and we could do it soon. Normally, only a medicinal company could have gotten to where we are now. I am very proud of how much we have already achieved with a core development team consisting of just three people supported by external experts. With the approved investigation plan in hand, we are now ready to start the next phase of the project. All we need is to finalize the funding”: Jes Trygved, MBA, Commercial project manager in Cessatech.